Main content
Top content
Genetic risk factors and early life stress interact to shape endophenotypes of affective disorders: neuroendocrine, cognitive and behavioural effects of this G x E interaction
Epidemiological studies have proven a strong impact of an individual’s genetic constitution on traits linked with affective disorders such as major depression. Through large scale genomic screening studies, certain genes or alleles have been associated with an increased risk of developing these disorders. However, genes never act in isolation but are embedded in organisms that develop and live in a complex environment. In consequence, the interaction between genetic and environmental factors brings about the phenotype observed in patients and animal models of affective disorders (see Figure 1). Exposure to early life stress is an acknowledged risk factor for the development of various affective disorders. Studies using an early life stress manipulation in rodents have shown that this experience contributes to a lasting dysregulation of the HPA axis, with impaired glucocorticoid (GC) signalling sensitivity and impaired negative feedback inhibition of the stress hormone system and behavioural alterations associated with depression. Childhood maltreatment in humans is also associated with impaired glucocorticoid-mediated feedback control of the HPA axis. This can induce persistent changes in the ability of the HPA axis to respond appropriately to stressors in adulthood, thereby increasing the susceptibility to depression.
In this very recently started research project we attempt to mimic the clinical situation of a genetic predisposition interacting with environmental stress during early life by exposing animals from the three breeding lines of our stress reactivity (SR) mouse model to an early life stress (ELS) paradigm. Our long-term aim is to gain a deeper insight into the mechanisms that are underlying the pathophysiological changes observed in depressed patients. We started investigating the effects of early life stress on emotionality, depression-like/stress-coping behaviours, and cognitive functions, as well as the effects on the reactivity and regulation of the HPA axis in our three SR mouse lines. In an initial step, we are testing the effects of early life stress (induced by limited availability of nesting and bedding material from PND 2 to 9) on cognition in a hippocampus-dependent spatial learning task (water cross-maze), followed by a tail suspension test as a behavioural measure of depressive tendency and a Dex/CRH test to probe HPA axis functions. To reveal the molecular processes associated with these changes we will analyze the expression of relevant proteins (receptors, neurotransmitters and neuromodulating peptides) in specific brain areas (e.g. the hippocampus, amygdala, and the hypothalamic paraventricular nucleus) of these animals.
Moreover, by applying pharmacological interventions (treatment with antidepressant drugs or receptor antagonists/agonists after the stress exposure) at a later stage of this research project we aim to elucidate fundamental mechanisms of antidepressant action.
Our long-term goal is to track down stress-induced alterations on a behavioural and neuroendocrine level to underlying molecular/epigenetic processes. Thus, with our study we aim at revealing the interaction between genetic predisposition and environmental factors in order to gain further insight into the aetiology and pathophysiology of affective disorders.
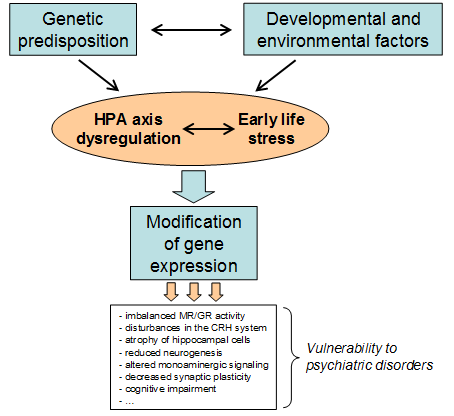
Interaction between genetic and environmental factors (G x E) can bring about phenotypes conferring vulnerability to psychiatric disorders through triggering changes in gene expression.

